研究揭示叶绿素合成途径中关键反应
来源:PNAS
作者:刘琳等
时间:2014-06-23
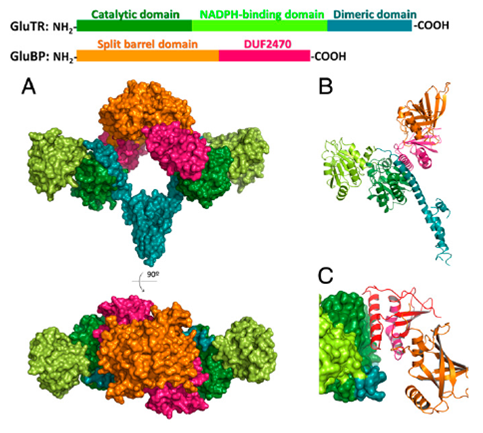
中科院植物所光生物学重点实验室刘琳研究组日前解析了拟南芥的谷氨酰-tRNA还原酶(GluTR)与其结合蛋白的复合物晶体结构。该结构中GluTR处于活性状态,反应的产物释放通道在结构中得到清楚的展现。实验分析发现,GluTR的活性受到其结合蛋白的正调控。
研究澄清了国际上长期以来关于GluTR的激活与调控方面的疑问,拓展了人们对GluTR调控多样性的认识,也为人们研究叶绿素合成调控提供了新线索。研究结果近日在线发表在《美国科学院院报》上。
据了解,叶绿素是植物光合作用吸收和传递光能的最主要色素,叶绿素的生物合成途径由一系列酶促反应完成。GluTR催化的NADPH对谷氨酰-tRNA的还原,是叶绿素合成途径的第一个关键限速反应。因此,GluTR的结构与功能研究对揭示叶绿素合成的调节机制具有重要意义。(来源:中国科学报 丁佳)
Crystal structure of Arabidopsis glutamyl-tRNA reductase in complex
with its stimulator protein
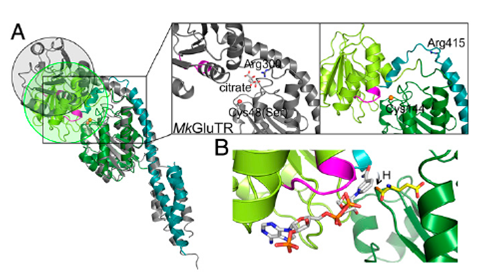
Abstract Tetrapyrrole biosynthesis in plants, algae, and most bacteria starts from the NADPH-dependent reduction of glutamyl-tRNA by glutamyl-tRNA reductase (GluTR). The GluTR-catalyzed reaction is the rate-limiting step, and GluTR is the target of multiple posttranslational regulations, such as heme feedback inhibition, for the tetrapyrrole biosynthetic pathway. A recently identified GluTR regulator, GluTR binding protein (GluBP), has been shown to spatially organize tetrapyrrole synthesis by distributing GluTR into different suborganellar locations. Here we report the complex structure of GluTR–GluBP from Arabidopsis thaliana. The dimeric GluBP binds symmetrically to the catalytic domains of the V-shaped GluTR dimer via its C-terminal domain. A substantial conformational change of the GluTR NADPH-binding domain is observed, confirming the postulated rotation of the NADPH-binding domain for hydride transfer from NADPH to the substrate. Arg146, “guarding the door” for metabolic channeling, adopts alternative conformations, which may represent steps involved in substrate recognition and product release. A coupled enzyme assay shows that GluBP stimulates GluTR catalytic efficiency with an approximate threefold increase of the 5-aminolevulinic acid formation rate. In addition, the GluTR activity can be inhibited by heme in a concentration-dependent way regardless of the presence of GluBP. A structural alignment indicates that GluBP belongs to a heme-binding family involved in heme metabolism. We propose a catalytic mechanism model for GluTR, through which photosynthetic organisms can achieve precise regulation of tetrapyrrole biosynthesis.
原文链接:http://www.pnas.org/content/111/18/6630.full.pdf+html




