研究揭示斑鸠霉素多环生物合成机制
来源:《应用化学》
作者:张长生等
时间:2014-06-16
记者日前从中科院南海海洋所获悉,该所热带海洋生物资源与生态重点实验室张长生研究团队首次揭示了抗肿瘤天然产物斑鸠霉素还原成环的多环生物合成机制。相关成果发表于《应用化学》杂志,并获Faculty of 1000推荐。
据介绍,PTM类化合物是一类广泛存在且活性多样的天然产物。其结构复杂,具有多环稠合的大环内酰胺结构,属于聚酮和非核糖体肽杂合抗生素。虽然科学家已发现PTMs基因簇,并推测出聚酮合酶/非核糖体肽合成酶组装PTM类化合物骨架的生源本质,但PTMs类化合物的多环形成机制仍是未解之谜。
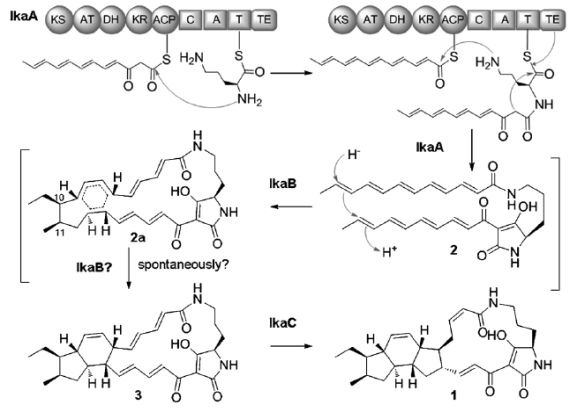
斑鸠霉素是PTMs家族的代表性化合物,其独特的结构和优良的抗肿瘤活性吸引了广泛关注。此次科研人员从珠江口沉积物来源的海洋链霉菌Streptomyces sp. ZJ306中发现了斑鸠霉素,并发现与其他PTMs化合物一致,斑鸠霉素的生物合成源于聚酮合成酶和非核糖体肽合成酶(PKS/NRPS)的杂合途径。
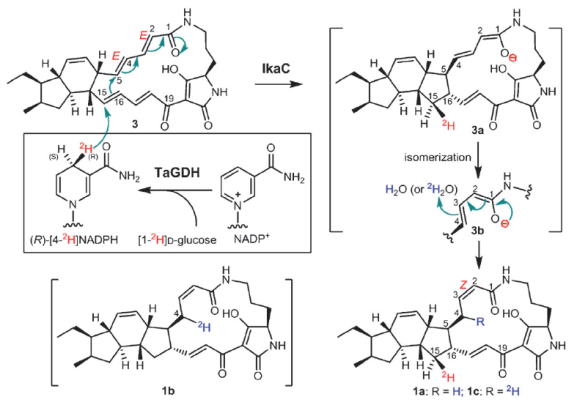
通过基因敲除及异源表达等技术,科学家确定了三个基因ikaABC足以介导斑鸠霉素的异源生物合成,同时初步阐明了ikaABC的功能及反应顺序。通过体外生化和巧妙的氘原子标记实验,这种独特的还原环化反应机制获得解析和证实。(来源:中国科学报 李洁尉 陈忠)
Mechanistic Insights into Polycycle Formation by Reductive Cyclization
in Ikarugamycin Biosynthesis
Abstract Ikarugamycin is a member of the polycyclic tetramate macrolactams (PTMs) family of natural products with diverse biological activities. The biochemical mechanisms for the formation of polycyclic ring systems in PTMs remain elusive. The enzymatic mechanism of constructing an inner five-membered ring in ikarugamycin is reported. A three-gene-cassette ikaABC from the marine-derived Streptomyces sp. ZJ306 is sufficient for conferring ikarugamycin production in a heterologous host. IkaC catalyzes a reductive cyclization reaction to form the inner five-membered ring by a Michael addition-like reaction. This study provides the first biochemical evidence for polycycle formation in PTMs and suggests a reductive cyclization strategy which may be potentially applicable in general to the corresponding ring formation in other PTMs.
原文链接:http://onlinelibrary.wiley.com/doi/10.1002/anie.201402078/pdf




