组合生物合成研究获重大突破
来源:《美国国家科学院院刊》
作者:István Molnár
时间:2014-08-21
本报北京7月24日电 记者胡其峰24日从中国农科院获悉,一项由中美科学家合作完成、针对聚酮化合物组合生物合成的研究近日获得重大突破;该研究成果可为新一代药物筛选提供新的候选化合物库,同时为揭示天然聚酮类化合物的程序化合成机制,奠定了重要理论基础,为新型药物的开发提供了新的技术途径。
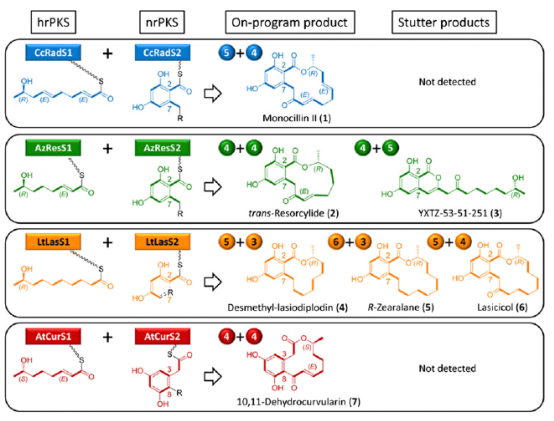
组合生物合成技术,是近年发展起来的一种扩展天然产物结构多样性、形成新的生物合成途径,从而产生非天然化合物的新方法。中国农科院生物技术研究所研究员林敏科研团队与美国亚利桑那大学自然资源和环境学院教授莫尔纳(Molnar)科研团队,针对目前已经研究清楚的四种天然苯二酚内酯聚酮化合物的模式生物合成途径,利用组合生物合成技术,实现一系列“非天然的”的聚酮类化合物的一步合成,这种“即插即用”的模块化方法,可应用于结构多样的全新化学物质的组合生物合成。
此项研究对于拓宽医药和农业生物活性物质的范围,提供了新策略和新途径,具有重要的理论研究与产业应用价值;相关论文已经在线发表在最近的美国科学院报(PNAS)上。(来源:光明日报 胡其峰)
Diversity-oriented combinatorial biosynthesis of benzenediol lactone scaffolds by subunit shuffling of fungal polyketide synthases
Abstract Combinatorial biosynthesis aspires to exploit the promiscuity of microbial anabolic pathways to engineer the synthesis of new chemical entities. Fungal benzenediol lactone (BDL) polyketides are important pharmacophores with wide-ranging bioactivities, including heat shock response and immune system modulatory effects. Their biosynthesis on a pair of sequentially acting iterative polyketide synthases (iPKSs) offers a test case for the modularization of secondary metabolic pathways into “build–couple–pair” combinatorial synthetic schemes. Expression of random pairs of iPKS subunits from four BDL model systems in a yeast heterologous host created a diverse library of BDL congeners, including a polyketide with an unnatural skeleton and heat shock response-inducing activity. Pairwise heterocombinations of the iPKS subunits also helped to illuminate the innate, idiosyncratic programming of these enzymes. Even in combinatorial contexts, these biosynthetic programs remained largely unchanged, so that the iPKSs built their cognate biosynthons, coupled these building blocks into chimeric polyketide intermediates, and catalyzed intramolecular pairing to release macrocycles or α-pyrones. However, some heterocombinations also provoked stuttering, i.e., the relaxation of iPKSs chain length control to assemble larger homologous products. The success of such a plug and play approach to biosynthesize novel chemical diversity bodes well for bioprospecting unnatural polyketides for drug discovery.
原文链接:http://www.pnas.org/content/early/2014/07/16/1406999111.full.pdf+html?with-ds=yes




