科学家发现两栖动物体内抗感染物质
来源:PNAS
作者:张云等
时间:2014-07-24
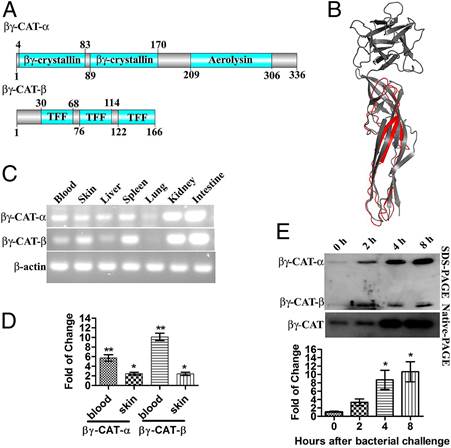
中科院昆明动物所张云课题组从两栖动物大蹼铃蟾中分离和克隆了第一个细菌毒素样蛋白和三叶因子复合物betagamma-CAT,动物体内模型证明其具有清除细菌、保护动物免受致命感染的功能。相关成果日前在美国《国家科学院院刊》发表。
天然免疫是机体的第一道防线,在抵御和清除病原微生物侵害中起着重要作用。病原微生物感染机体依赖毒力因子,其中孔道形成毒素是最大的一类由致病菌产生的蛋白毒力因子,能插入细胞膜形成通道引起细胞损伤。目前,人们发现细菌毒素样蛋白广泛存在于各种动植物中,但尚不清楚它们的生物学功能。
张云等从两栖动物大蹼铃蟾中分离和克隆出betagamma-CAT,并发现该蛋白质复合物的表达调控与微生物感染密切相关。betagamma-CAT的作用机制在于通过宿主细胞受体介导的该蛋白复合物内吞,溶酶体膜寡聚化和通道形成,造成细胞炎症小体的激活,从而迅速有效地激发体内天然免疫响应清除体内微生物的感染。
该研究为阐明机体调控天然免疫响应的策略和分子途径等问题提供了新思路和线索,同时对深入揭示感染和炎症相关疾病的发生机理、研发新的疾病治疗策略和药物具有重要意义。(来源:中国科学报 张雯雯)
Host-derived, pore-forming toxin–like protein and trefoil factor complex protects the host against microbial infection
Abstract Aerolysins are virulence factors belonging to the bacterial β-pore–forming toxin superfamily. Surprisingly, numerous aerolysin-like proteins exist in vertebrates, but their biological functions are unknown. βγ-CAT, a complex of an aerolysin-like protein subunit (two βγ-crystallin domains followed by an aerolysin pore-forming domain) and two trefoil factor subunits, has been identified in frogs (Bombina maxima) skin secretions. Here, we report the rich expression of this protein, in the frog blood and immune-related tissues, and the induction of its presence in peritoneal lavage by bacterial challenge. This phenomena raises the possibility of its involvement in antimicrobial infection. When βγ-CAT was administrated in a peritoneal infection model, it greatly accelerated bacterial clearance and increased the survival rate of both frogs and mice. Meanwhile, accelerated Interleukin-1β release and enhanced local leukocyte recruitments were determined, which may partially explain the robust and effective antimicrobial responses observed. The release of interleukin-1β was potently triggered by βγ-CAT from the frog peritoneal cells and murine macrophages in vitro. βγ-CAT was rapidly endocytosed and translocated to lysosomes, where it formed high molecular mass SDS-stable oligomers (>170 kDa). Lysosomal destabilization and cathepsin B release were detected, which may explain the activation of caspase-1 inflammasome and subsequent interleukin-1β maturation and release. To our knowledge, these results provide the first functional evidence of the ability of a host-derived aerolysin-like protein to counter microbial infection by eliciting rapid and effective host innate immune responses. The findings will also largely help to elucidate the possible involvement and action mechanisms of aerolysin-like proteins and/or trefoil factors widely existing in vertebrates in the host defense against pathogens.
原文链接:http://www.pnas.org/content/111/18/6702.full.pdf+html?with-ds=yes




