

衍生自迷幻蘑菇的一种致幻药能够被用来治疗抑郁症,对这种疗法进行的首个安全性研究日前得出了上述结论。英国科学家在一个小规模试验中,尝试用迷幻蘑菇中一种具迷幻作用的成分治疗抑郁症患者,取得一定效果。未来如果能通过更大规模的临床试验验证其效果,或许能为这类心理疾病的治疗开辟新思路。
伦敦帝国理工学院的研究人员让12名受试者服用了裸盖菇素,后者是迷幻蘑菇中的一种活性成分。所有这些受试者在临床上都有很长时间的抑郁症患病史——平均17.8年。同时所有患者对于抑郁症标准疗法都没有响应,例如选择性血清素再吸收抑制剂(SSRIs)或电休克疗法。
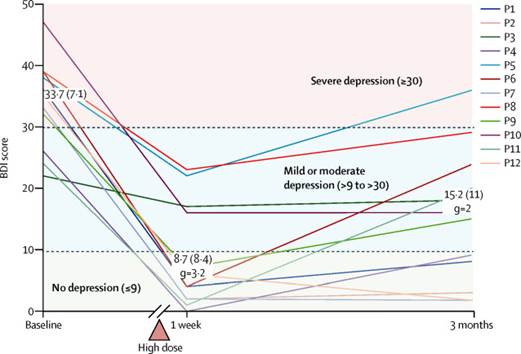
在接受了口服剂量的裸盖菇素1周后,所有患者的症状都得到了显著改善。3个月后,其中5位患者得到了完全缓解。
研究人员在最新出版的《The Lancet Psychiatry》杂志上报告了这一研究成果。该项研究的第一作者、帝国理工学院神经精神药理学家Robin Carhart-Harris表示:“在当前背景下,这是一种相当了不起的可获得的疗法。”
而SSRIs的相同剂量缓解率大约为20%。
目前对抑郁症的治疗主要通过抗抑郁药物以及认知行为疗法来进行,但这类治疗方案并不适合所有的抑郁症患者,为此有必要寻找新的治疗途径。
研究人员介绍说,迷幻蘑菇中的成分裸盖菇素能够作用于人体内的一种神经传递物质血清素,因此被医学界视为一种很有潜力的候选药物。但裸盖菇素这类具有致幻作用的物质也容易引起一些不良反应,因此使用要非常小心。
在研究人员招募的12名患不同程度抑郁症的患者中,男女各占一半。他们相隔7天服用了两次一定剂量的裸盖菇素。
结果显示,在整个试验观察期内,药物并没有在这些患者身上产生不良反应,所有患者的症状都有一定程度缓解,其中7人在治疗结束后的3个月里持续好转,另外5人的症状缓解时间更长。
该项研究通讯作者、帝国理工学院神经精神病理学家David Nutt说,从试验结果来看,如果经过严格评估后酌量使用裸头草碱,能够缓解抑郁症患者的症状。
不过研究人员也指出,目前的试验规模还比较小,需要进一步大规模临床试验才能验证这种成分是否安全有效。
Carhart-Harris说,这种成分本身具有很强的致幻作用,并不建议普通人随意使用它自行治疗抑郁症,应在专业指导下进行相关治疗。
该研究的作者并不认为裸盖菇素能够成为治疗抑郁症患者的最后手段。Carhart-Harris说:“我们的结论比这更冷静,我们只是简单地说,这是可行的。”他表示:“我们可以给抑郁症患者使用裸盖菇素,他们可以忍受它,它是安全的。这给了我们一个治疗有效性的初步印象。”
证明裸盖菇素的安全性并不是一个简单的工作。迷幻蘑菇在英国被列为非法毒品——这是最严重的一个类别,还包括海洛因及可卡因。
批准该项研究的伦理委员会非常担心受试者会经历延迟发作的精神病症状,因此要求对受试者在接下来的3个月中进行随访。Nutt说:“这是前所未有的。”
美国新墨西哥州圣达菲市海福特研究所的科学家之前已经开始研究裸盖菇素如何能够减轻癌症晚期患者的抑郁和焦虑,但这是第一个专门分析裸盖菇素如何用于治疗抑郁症的研究。(来源:中国科学报 赵熙熙)
Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study
Abstract Background Psilocybin is a serotonin receptor agonist that occurs naturally in some mushroom species. Recent studies have assessed the therapeutic potential of psilocybin for various conditions, including end-of-life anxiety, obsessive-compulsive disorder, and smoking and alcohol dependence, with promising preliminary results. Here, we aimed to investigate the feasibility, safety, and efficacy of psilocybin in patients with unipolar treatment-resistant depression. Methods In this open-label feasibility trial, 12 patients (six men, six women) with moderate-to-severe, unipolar, treatment-resistant major depression received two oral doses of psilocybin (10 mg and 25 mg, 7 days apart) in a supportive setting. There was no control group. Psychological support was provided before, during, and after each session. The primary outcome measure for feasibility was patient-reported intensity of psilocybin's effects. Patients were monitored for adverse reactions during the dosing sessions and subsequent clinic and remote follow-up. Depressive symptoms were assessed with standard assessments from 1 week to 3 months after treatment, with the 16-item Quick Inventory of Depressive Symptoms (QIDS) serving as the primary efficacy outcome. This trial is registered with ISRCTN, number ISRCTN14426797. Findings Psilocybin's acute psychedelic effects typically became detectable 30–60 min after dosing, peaked 2–3 h after dosing, and subsided to negligible levels at least 6 h after dosing. Mean self-rated intensity (on a 0–1 scale) was 0·51 (SD 0·36) for the low-dose session and 0·75 (SD 0·27) for the high-dose session. Psilocybin was well tolerated by all of the patients, and no serious or unexpected adverse events occurred. The adverse reactions we noted were transient anxiety during drug onset (all patients), transient confusion or thought disorder (nine patients), mild and transient nausea (four patients), and transient headache (four patients). Relative to baseline, depressive symptoms were markedly reduced 1 week (mean QIDS difference −11·8, 95% CI −9·15 to −14·35, p=0·002, Hedges' g=3·1) and 3 months (−9·2, 95% CI −5·69 to −12·71, p=0·003, Hedges' g=2) after high-dose treatment. Marked and sustained improvements in anxiety and anhedonia were also noted. Interpretation This study provides preliminary support for the safety and efficacy of psilocybin for treatment-resistant depression and motivates further trials, with more rigorous designs, to better examine the therapeutic potential of this approach.
原文链接:http://www.sciencedirect.com/science/article/pii/S2215036616300657



