
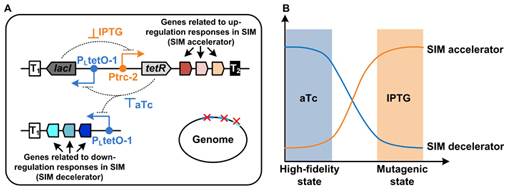
图:胁迫诱导突变模块的设计思路。A:采用经典的双稳态开关,对引发和抑制胁迫诱导突变的基因进行双稳态控制。B:加入不同的诱导剂,使细胞在正常态和突变态之间切换。
胁迫抗性是工业微生物的重要属性之一。微生物的胁迫抗性是多基因控制的复杂生理性状,单基因改造方法很难有效发挥作用。而针对多基因的化学/物理诱变、转录因子改造等方法,均采用“先突变后筛选”的策略,需要频繁的人工介入,导致整个改造过程不连续且效率低。
为了快速提高工业微生物对不同环境胁迫的抗性,中国科学院微生物研究所李寅课题组已开发出基因组复制工程辅助的连续进化技术GREACE(Luan et al. Biotechnology for Biofuels 2013, 6:137)。其基本原理是降低基因组复制过程的保真性,提高突变频率,实现在给定环境胁迫条件下的“边突变边筛选”,达到连续高效进化的目的。相关系统已提供给国内外十多个科研机构使用。
在自然界的胁迫条件下,极少量微生物细胞会通过一系列的生理调控,进入一种基因组复制突变率极高的超突变态。受这一现象的启发,李寅课题组的研究人员采用双稳态开关,创建了一个胁迫诱导突变(stress-induced mutagenesis)的进化模块。将该模块植入大肠杆菌后,加入诱导剂IPTG可引发胁迫诱导突变,使细胞跃迁到突变态,从而适应环境胁迫。当达到进化目标后,加入诱导剂aTc抑制胁迫诱导突变,使细胞回归到正常态,保持进化后的性状稳定遗传。
在这一设计思想指导下,研究人员通过10个周期的IPTG/aTc切换,在2个半月内就将大肠杆菌对丁醇的最低耐受浓度提高了56%,实现了复杂生理性状的人工控制进化。研究结果为进化工程增加了一种可控进化新方法,也为胁迫诱导突变理论提供了新的实验证据。(来源:中国科学院微生物研究所)
Development of a stress-induced mutagenesis module for autonomous adaptive evolution of Escherichia coli to improve its stress tolerance
Abstract Background Microbial tolerance to different environmental stresses is of importance for efficient production of biofuels and biochemical. Such traits are often improved by evolutionary engineering approaches including mutagen-induced mutagenesis and successive passage. In contrast to these approaches which generate mutations in rapidly growing cells, recent research showed that mutations could be generated in non-dividing cells under stressful but non-lethal conditions, leading to the birth of the theory of stress-induced mutagenesis (SIM). A molecular mechanism of SIM has been elucidated to be mutagenic repair of DNA breaks. This inspired us to develop a synthetic SIM module to simulate the mutagenic cellular response so as to accelerate microbial adaptive evolution for an improved stress tolerance.
Results A controllable SIM evolution module was devised based on a genetic toggle switch inEscherichia coli. The synthetic module enables expression and repression of the genes related to up- and down-regulation responses during SIM in a bistable way. Upon addition of different inducers, the module can be turned on or off, triggering transition to a mutagenic or a high-fidelity state and thus allowing periodic adaptive evolution. Six genes (recA, dinB, umuD, ropS, ropE, and nusA) in the up-regulation responses were evaluated for their potentials to enhance the SIM rate. Expression of recA, dinB, or ropSalone increased the SIM rate by 4.5- to 13.7-fold, whereas their combined expression improved the rate by 31.9-fold. Besides, deletion of mutL increased the SIM rate by 82-fold. Assembly of these genes into the SIM module in the mutL-deletion E. coli strain elevated the SIM rate by nearly 3000-fold. Accelerated adaptive evolution of E. coliequipped with this synthetic SIM module was demonstrated under n-butanol stress, with the minimal inhibitory concentration of n-butanol increasing by 56 % within 2.5 months.
Conclusions A synthetic SIM module was constructed to simulate cellular mutagenic responses during SIM. Based on this, a novel evolutionary engineering approach—SIM-based adaptive evolution—was developed to improve the n-butanol tolerance of E. coli.
原文链接:http://www.biotechnologyforbiofuels.com/content/pdf/s13068-015-0276-1.pdf



